The mysterious Oxford Electric Bell, as the unusual object has been called, is counted among the world’s oldest experiments, though it may not have been an experiment originally. No one seems to be entirely clear exactly what the battery, which is the source of power for the entire apparatus for nearly 18 decades now, is composed of. There is a note on the bell that states the device was supposedly set up in 1840. However, some accounts at Oxford University say the bell could have been started even earlier, in 1825.
The bell was the product of the company Watkins and Hill that dealt with producing various instruments, based in London. It eventually ended up in the university quarters at some point during the mid-19th century after it was brought there by a University of Oxford physics professor, Robert Walker, the Smithsonian writes.
The battery powers a small ball made of metal that quickly oscillates between the first and the second installed bell, and after so many decades, it is hard to believe it is still functioning. The device has been distinguished for holding a Guinness World Record for having the most durable battery in the world. Thanks to the battery, the bell would have rung roughly 10 billion times, and probably more than that, calculations from the university suggest.
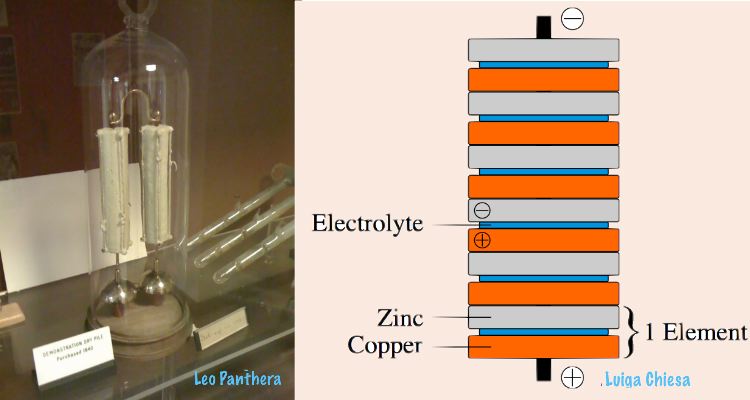
Nobody wants to open the apparatus at this point, as that could potentially harm it and "ruin an experiment to see how long it will last," Motherboard writes. The battery is made of dry piles assumed to be of a similar composition as some of the world’s first electricity batteries, an invention of Italian-born physicist Giuseppe Zamboni from the early 19th century.
Because movement of the hanging metal clapper that moves between the two bells makes use of electrostatic forces, the system can continuously run using only low currents of electricity. The battery likely makes use of alternate discs or layers of silver, zinc, and possibly other materials. These are insulated with sulfur, protecting them from moisture for example.
However, what’s precisely inside the battery is one of the most burning questions for those who are dying to know all details of the mysterious mechanism. A former researcher at the Clarendon Laboratory, A. J. Croft, described the apparatus in a 1984 paper for the European Journal of Physics. "What the piles are made of is not known with certainty, but it is clear that the outer coating is of sulfur, and this seals in the cells and the electrolyte," he notes. He continues to explain how similar the piles of the Oxford bell battery should be with the ones that had been produced by the Italian physicist Zamboni.
Finding the ultimate answers that veil the bell apparatus with mystery is bound to happen as the battery ultimately wears out and the entire mechanism comes to a halt due to its extensively prolonged lifetime. Whether researchers will find the experiment useful for developing new types of batteries, the answer is no.
By this point, so many new types of batteries have been devised and produced that some of the latest proposed designs sound futuristic. According to the World Economic Forum, scientists at the University of Bristol Cabot Institute are working on a diamond battery made from nuclear waste. The lifetime of this battery should be incredible, and if scientists are correct, it should work for thousands of years.
A bell that is displayed at the Clarendon Laboratory at the University of Oxford in England has been relentlessly ringing for some 178 years if the math is correct. But don’t worry, no matter how annoying the thought of ringing that never ends, this bell is hardly heard, shielded by quite thick layers of glass.




